The Metal Sorption Study began in the summer of 2013 and investigates the ability of biochar to absorb metals from water. In 2014, Langara College was awarded a $25,000 grant by the National Science and Engineering Research Council (NSERC), under the College and Community Innovation Program. The Applied Research and Development grant (ARD) will provide valuable funding for further work on this research.
The goal of this new research will be exploring the potential of using biochar, which has the ability to adsorb metal ions, as a filter media in wastewater cleaning technologies. Biochar made from different sources, and chemically modified biochar, will be tested in an effort to find the optimum metal adsorption characteristics. Sveinson and his team will work with industry partner McCue Engineering Contractors to ensure the work has direct relevance to industrial applications.
A typical metal sorption experiment is conducted in the Langara chemistry lab.
Rationale
There are many industries, such as mining, that create water that is contaminated with metals. Sometimes these metals are a danger to the environment, and other times they interfere with the water processing. Biochar is known to adsorb metals. Can we make a biochar that can clean up metal contaminated water at an industrial scale?
Methods
In this study we are making water samples spiked with metals, exposing the water to various biochar types, and measuring the metal concentration in the water after exposure. After identifying promising biochars, we will work with actual waste water samples
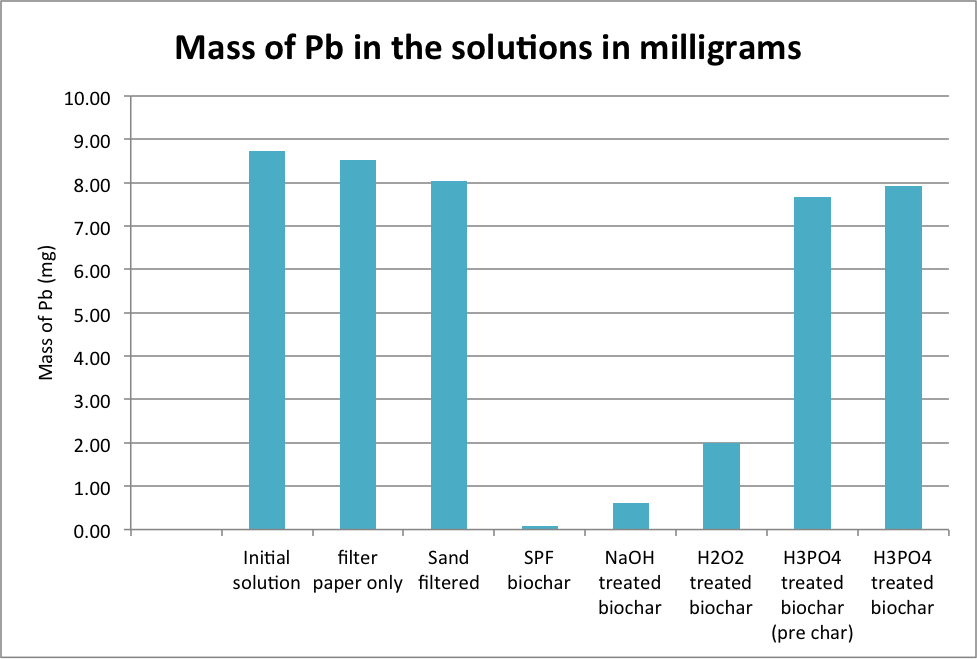
Results
This study is in the early stages. So far we have worked only with iron, and found that “standard” biochar does not adsorb much iron, but our modified biochar can adsorb up to 30% of the iron from the solution. The preliminary results showed:
- The biochar had a large ability to remove lead. Starting with a 100 ppm solution, the lead concentration was reduced by 99%. The modified biochars were less effective.
- The phosphoric acid treated biochar had a moderate ability to reduce iron. Starting with a 1000 ppm solution, the iron concentration was reduced by 14%. The initial concentrations were high though, and the effect may be more pronounced in lower concentration solutions.

Partners
Diacarbon Energy Inc., McCue Environmental Contracting Inc.

